View count:
17331
Notices on Submissions for Research Ethics Review
1.Personnel involved in the project must provide proof of ethics-related training hours.(1) Principal Investigator (PI): Must provide proof of 2 hours of ethics-related training per year or 6 hours within three years.
(2) Co-Principal Investigators, Collaborators, and Assistants: Must provide proof of 1 hour of ethics-related training per year or 3 hours within three years.
For recent education and training information, please visit https://ntnurec.ntnu.edu.tw/zh_tw/resource/trainingresources
2.The project's nature and recommendations for review applications
(1) A plan must be formulated before conducting human body research, and the research project must pass the review of the Research Ethics Review Committee before it may be implemented. (Human body research: refers to research involving obtaining, investigating, analyzing, or using human specimens or an individual person's biological behavior, physiological, psychological, genetic, or medical information.)
(2)If it is a human subject research project, since there are no mandatory requirements in the law, it is recommended to submit an application and pass the Committee’s review before implementing the research project.
(3)In order to protect research participants and promote academic research and development, it is recommended to pass the review by the Research Ethics Review Committee before implementing research projects that involve the following matters:
A.Research projects that recruit participants.
B.Research projects that use personal information that can be used to directly or indirectly identify participants.
C.Research results will be submitted to an international academic journal.
D. Regulations of the organization providing subsidies (e.g., NSTC, MOHW) or data (e.g., National Health Insurance Research Database) are submitted for review.
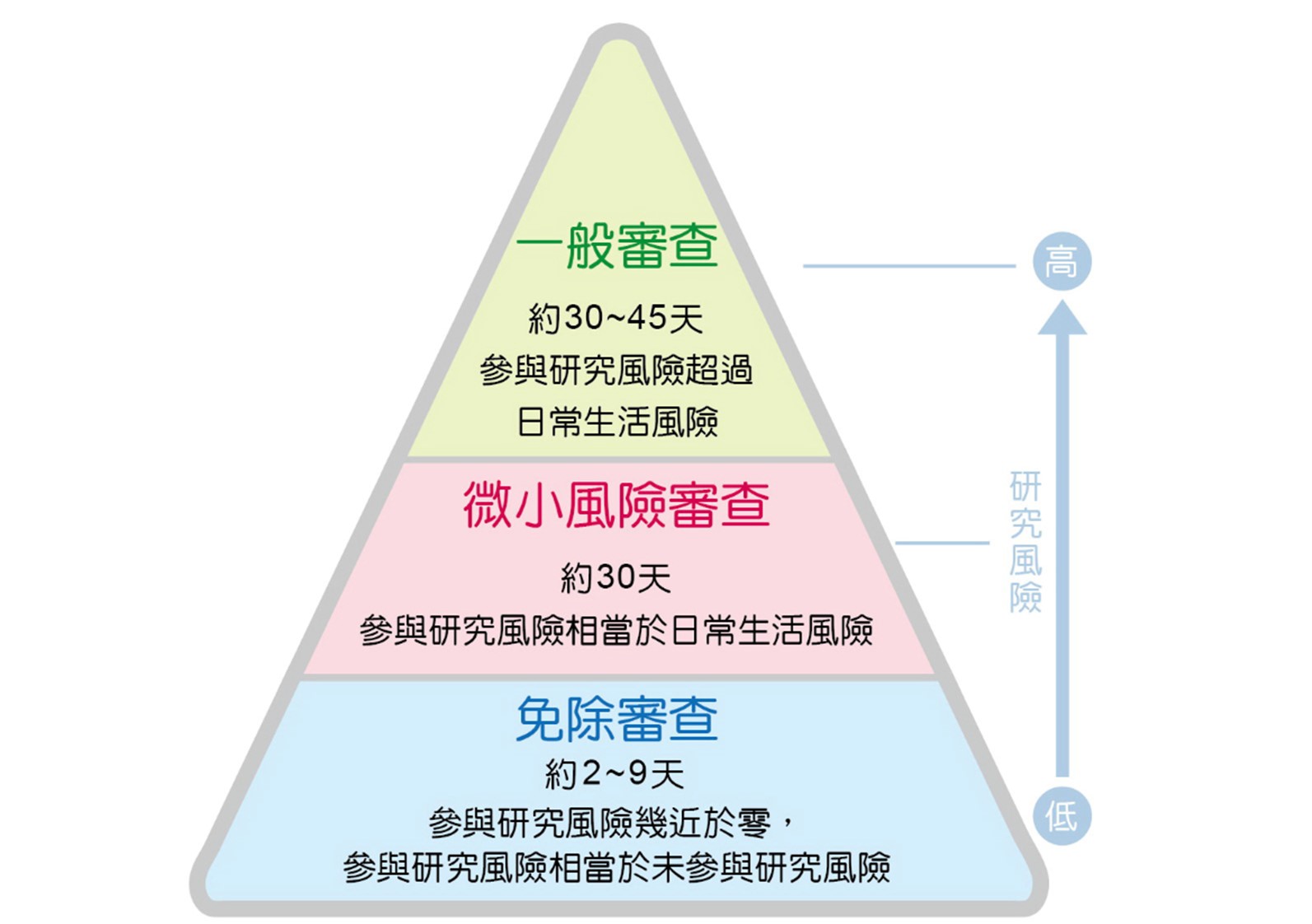
3.The committee provides the "Exemption from Review Cases - Principal Investigator's Self-Evaluation System," which is highly encouraged for use before submitting for review
"Exemption from Review Cases - Principal Investigator's Self-Evaluation System":
(1) You can use the system to check the conditions of the research subjects, research design, and other relevant details.
(2) This system will assist you in determining whether your human or human-related research falls within the exemption from review (based on the Ministry of Health and Welfare's Letter No. 1010265075 and related regulations).
(3) The system is intended to assist the Principal Investigator/research team in preliminary evaluation. The actual review outcome is subject to the final decision of the review committee.
4.Recruitment advertisements must include the statement "(Reposting without content modification is not allowed)"
(1) In accordance with the "Principles for Recruiting Participants in Clinical Trials."
(2) Please include the statement "Approved by the Research Ethics Committee of National Taiwan Normal University, reposting without content modification is not allowed" in the recruitment advertisement, along with the version and date of the document.
5.For research involving Indigenous peoples, the proposal must be submitted to the Indigenous Peoples Cultural Development Center for review
(1) In accordance with the "Human Subjects Research Act" §15:
1. For research purposes involving Indigenous peoples, in addition to complying with Articles 12 to 14, consultation and consent from the respective Indigenous groups must be obtained. This also applies to the publication of research results.
2. The consultation, consent, agreements regarding commercial interests, and their applications, as mentioned in the previous paragraph, shall be determined jointly by the central competent authority for Indigenous peoples and the responsible authorities.
(2) Indigenous Peoples Council:
Consultation and obtaining consent from Indigenous peoples for human research projects (Cultural Development Center).
6.For any questions or assistance, please contact the University's Research Ethics Committee at (02-77491903).


